

 |
 |
|
|---|---|---|
| I & C Technical Guides | ||
Many devices have been developed to grow biofilms in the lab for different purposes. For our purpose, it was essential to use material that permits fluorescent light penetration and does not have autofluorescence, i.e. glass with high optical properties. The next point to consider is whether you require a static biofilm or a biofilm grown in a flow cell. For mutagenesis screens for example the static biofilm will be more suitable. In contrast, to analyze biofilm formation the flow cell may provide valuable additional information. For our long term goals, we wanted to be able to analyze biofilms in a flow cell in real time.
Factors governing flow cell choice
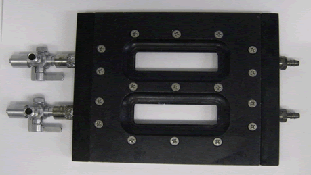
Based on these requirements, we chose to use the FC-281 flow cell from Biosurface Technologies shown below.
 |
|---|
Modifications to the flow cell
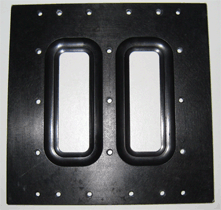
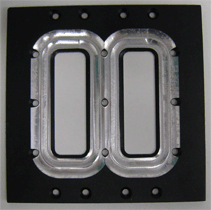
The microscope stage-mounting tab was removed and modified with a widened viewing window (see figure below) to facilitate access of the flatter, wider Plan-Apochromat 63x /1.4NA oil DIC objective and other high NA lenses and retain space for movement in the X,Y plane. Biosurface Technologies carried out this modification so any similar adaptation of the product is accessible to all research groups.
 |
 |
|---|---|
Original window |
Modified window |
Set up
Our set up involves placing the flow cell in an incubator, either on the microscope stage or as shown here in the lab during biofilm establishment (left hand image). The media was supplied at a controlled temperature using a water bath. A bubble trap (centre image) was used to limit air bubbles entering the flow cell. A highly accurate low speed Watson-Marlow 250U pump was used with 3.2mm diameter tubing (1.6mm bore, 1.6mm wall) for the whole system.
 |
 |
 |
|---|
| Imaging and Cytometry Laboratory Technology Facility, Department of Biology University of York, PO Box 373 York, YO10 5YW, UK |
Last modified on 3 August 2009 by Jo Marrison |